

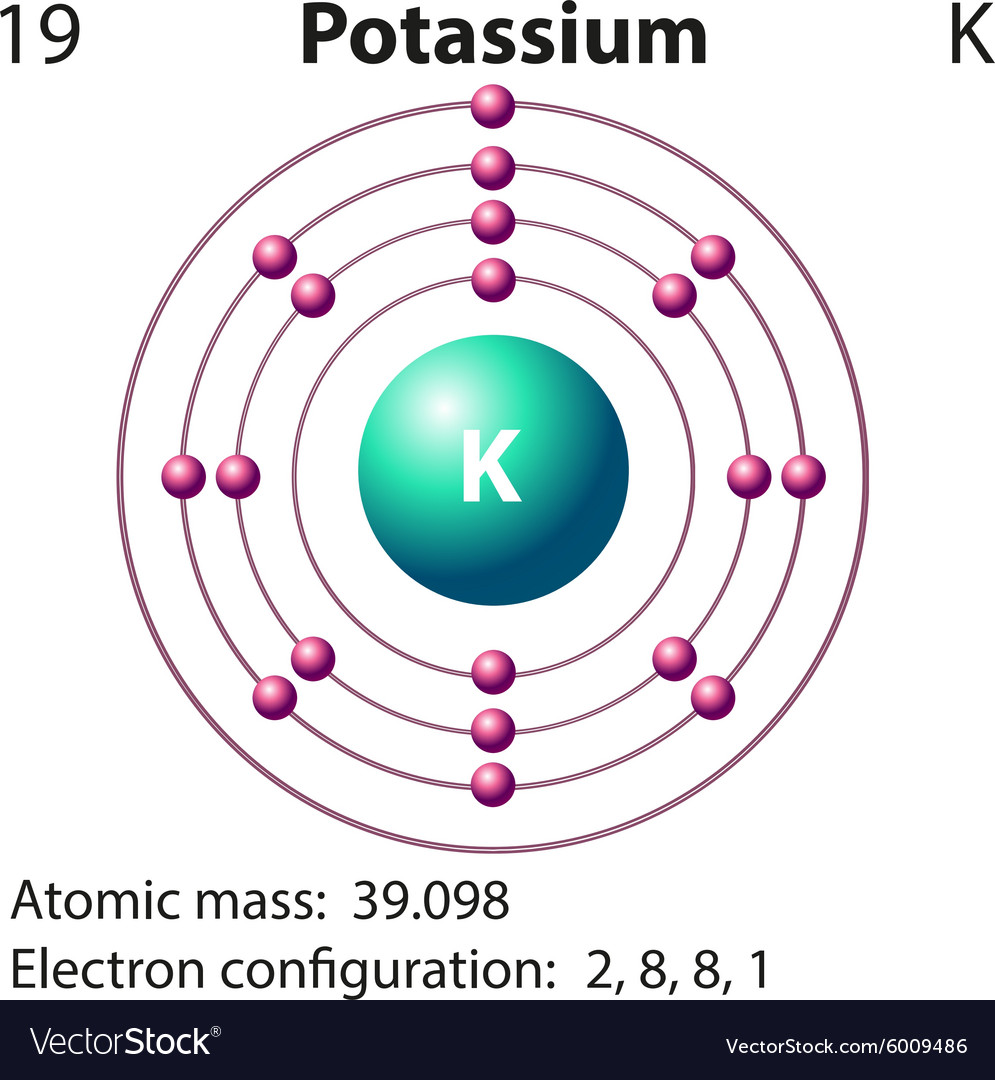
How do you write the electron configuration for Potassium? The electronic configuration of Potassium will be 1s2 2s2 2p6 3s2 3p6 4s1. Up to date, curated data provided by Mathematica s ElementData function from Wolfram Research, Inc. What is the electronic configuration of Potassium 19? What is the boiling Point of Potassium in Kelvin?īoiling Point of Potassium in Kelvin is 1032 K. Melting Point of Potassium in Kelvin is 336.53 K. What is the melting Point of Potassium in Kelvin? What is the boiling Point of Potassium?īoiling Point of Potassium is 1032 K. Potassium has 19 electrons out of which 1 valence electrons are present in the 4s1 outer orbitals of atom. How many valence electrons does a Potassium atom have? The element Potassium was discovered by H. It is located in group 1 and period 4 in the modern periodic table. Potassium is the 19 element on the periodic table. Potassium is a chemical element with the symbol K and atomic number 19. What is the position of Potassium in the Periodic Table? Potassium is a chemical element with symbol K and atomic number 19. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Potassium is 4s1. What is the abbreviated electronic configuration of Potassium? The atomic number of each element increases by one, reading from left to right. Period A horizontal row in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell.
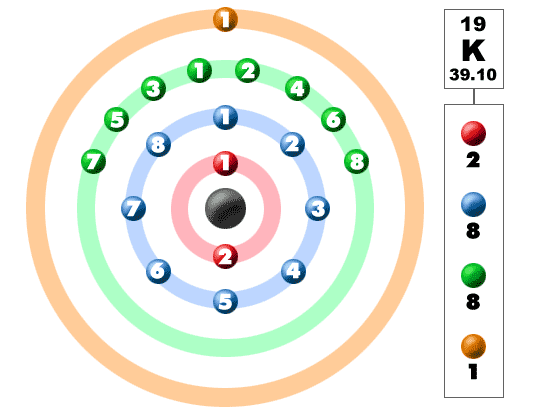
The electronic configuration of Potassium is 1s2 2s2 2p6 3s2 3p6 4s1. 39.098 Glossary Group A vertical column in the periodic table. What is the electronic configuration of Potassium? Potassium Thermal Properties - Enthalpies and thermodynamics Optical Properties of Potassium Refractive IndexĪcoustic Properties of Potassium Speed of Sound Potassium Magnetic Properties Magnetic Type Potassium Heat and Conduction Properties Thermal Conductivity
Refer to table below for the Electrical properties ofPotassium Electrical Conductivity
Hardness of Potassium - Tests to Measure of Hardness of Element Mohs Hardness Answer choice (C) is the correct answer.Refer to below table for Potassium Physical Properties DensityĠ.856 g/cm3(when liquid at m.p density is $0.828 g/cm3) So the alkali metal that will have the smallest atomic radius is lithium. So the atomic radius generally increases down a group in the periodic table. Higher energy electron shells are located further from the nucleus. Potassium is an alkali metal in group 1, period 4, and. Potassium that down a group, there are an increasing number of electron shells thatĪre occupied by electrons. The stability of an elements outer (valence) electrons determines its chemical and physical properties. We can see from these diagrams of the electron configuration of lithium, sodium, and So the radius increases.ĭown a group, electrons are located in increasingly higher energy shells. However, the atomic radius trend is not as simple as there are more subatomic This occurs because the atoms have more protons, neutrons, and electrons. We know that as we go down a group, the mass of the elements increases. The question wants us to understand the trend in atomic radius down a group. They include the elements lithium, sodium, potassium, rubidium, and cesium. The alkali metals are found in group one of the periodic table. Group? (A) Potassium, (B) rubidium, (C) lithium, (D) cesium, (E) sodium. Which of the following elements has the smallest atomic radius in the alkali metal


 0 kommentar(er)
0 kommentar(er)
